Our Products
Complete solutions for pharmaceutical production

VHP bio-decontamination cycle development
Our VHP® sterilisation cycle consists of four controlled phases: preconditioning, conditioning, decontamination and aeration.
Cape Europe activities include:
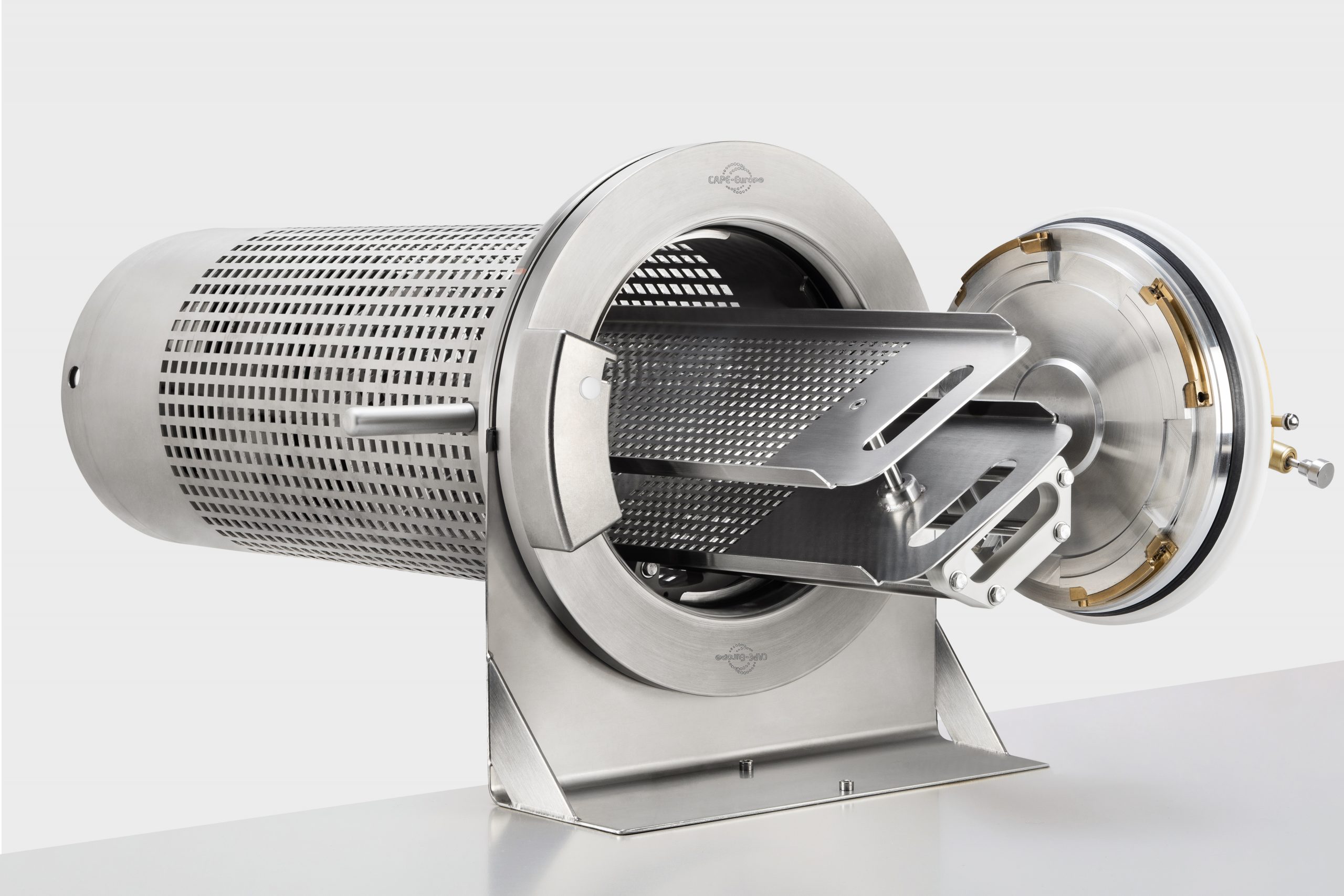
- Manufacture and supply of standard and bespoke rapid transfer ports (RTPs) 100% compatible with the Getinge La Calhène DPTE®.
- Design, supply and validation of bespoke products for isolator technology and Vaporised Hydrogen Peroxide bio-decontamination.
- IQ, OQ and PQ qualification and validation services for isolators, biosafety cabinets and controlled environments/separative enclosures.
- Design, supply and validation of complete Bio-decontamination systems solutions for Research Laboratories, Pharmaceutical and API facilities.
- VHP bio-decontamination cycle development and qualification.
- Servicing and planned maintenance programmes for Isolator and VHP equipment.
- Isolator technology operator training packages